Babies thrive under a ketogenic metabolism
Some people, even some scientists who study ketogenic metabolism, have the idea that ketogenesis is somehow abnormal, or exceptional; an adaptation for emergencies only. We disagree. One reason we think a ketogenic metabolism is normal and desirable, is that human newborns are in ketosis. Despite the moderate sugar content of human breast milk, breastfeeding is particularly ketogenic. This period of development is crucial, and there is extensive brain growth during it. Although the composition of breast milk can be affected by diet [1], it is reasonable to assume that breast milk has always been ketogenic, and this is not an effect of modernisation.
When the brain is in its period of highest growth, and when the source of food is likely to be close to what it evolved to be for that period, ketones are used to fuel that growth.
If nothing else, this suggests that learning is well supported by a ketogenic metabolism. It is also consistent with the ability of ketogenic diets to treat a variety of seemingly unrelated brain disorders and brain trauma.
In brief
- Newborn infants are in ketosis. This is their normal state.
- Breastfeeding is particularly ketogenic (compared to formula feeding).
- Breastfeeding longer (up to a point) is associated with better health outcomes.
- This suggests the hypothesis that weaning onto a ketogenic diet would be healthier than weaning onto a high-carb diet.
Mark up ours
Human babies are in ketosis
Soon after birth, human babies are in ketosis, and remain so while breastfeeding [2]. They use ketones and fats for energy and for brain growth.
When this has been studied, in the first couple of hours after birth, babies aren’t immediately in ketosis. There is a short delay [3]. During that brief period before ketogenesis starts, lactate (confusingly not to do with lactation) becomes an important fuel to suppport the brain [4]. Some researchers speculate that this delay in ketogenesis could be because of a limited supply of carnitine, which is supplied by milk, but they also note that glycogenolysis and gluconeogenesis (the process by which glucose is made out of protein) are not active immediately [5]. Therefore, it could simply be the case that ketogenesis takes time to get started. In other words, it may just be keto-adaptation.
Note, though, that the mothers of these babies were unlikely to have been ketogenic. As it happens, if the mother is in ketosis (as has been studied through fasting), ketone bodies will pass through the placenta and be used by the fetus [5], [6]. At the same time, gluconeogenesis is induced in the liver of the fetus, likely as a result of the insulin-to-glucagon ratio [7], [8]. Therefore, it is possible that the fetus of a ketogenic mother would already be independently ketogenic at birth.
Breastfeeding is probably healthy
Many positive associations between exclusive breastfeeding for at least 3-6 months and the later health of the child have been reported. For example, intelligence has been positively correlated with length of time breastfeeding. The data is conflicting and prone to confounds [9], although we found a few studies that appear to have addressed those confounds and still showed an effect [10], [11], [12]. There have also been correlations found between breastfeeding and protection from developing diseases, such as asthma and allergies [13], type 1 and type 2 diabetes [14], and epilepsy [15].
Observational correlations are good sources of hypotheses, but can’t establish causality. Unfortunately, these hypotheses are hard to test. We suppose that breastfeeding is healthy mainly because we clearly evolved to breastfeed.
Breastfeeding is ketogenic
The medical focus in the 20th century was heavily influenced by the discovery of micronutrients, and because of this, we have been looking for the secret of the healthfulness of breast milk by examining what nutrients it contains. However, one significant difference between breastfeeding infants and those drinking formula is that they are in deeper ketosis [16]. It is not known why. It could be a property of the milk, or something else about the feeding. In any case, regardless of mechanism, the fact is that breastfeeding is more ketogenic. It is possible that the reason that longer breastfeeding is generally associated with better health, is because it represents a longer time in ketosis.
Summary
- The period in which human brains grow the most, and in which food is least likely to be different from evolutionary conditions, is a ketogenic period. This suggests that a ketogenic metabolism is excellent for learning and development.
- Breastfeeding in humans is particularly ketogenic. We hypothesise that the positive associations between health and longer breastfeeding may be due to extending the period of ketosis in infancy.
- A related hypothesis we offer is that extending the period of ketosis after breastfeeding, by weaning onto ketogenic foods such as homemade broth [*] and fatty meat, rather than cereal, fruit, and starchy vegetables, would further promote brain development and reduce risk of disease.
[*] Homemade, because it is rich in fat, unlike the boxed varieties which have almost none.
References and notes
1. Evidence type: review
[Dietary Triacylglycerol Structure and Its Role in Infant Nutrition
Sheila M. Innis
Adv Nutr May 2011 Adv Nutr vol. 2: 275-283, 2011
“The fatty acids needed by the mammary gland for synthesis of TG for secretion in milk are obtained by uptake of fatty acids from plasma and de novo synthesis in the mammary gland (7). Fatty acid synthesis in the mammary gland, however, is unusual. Commencing with acetyl CoA, malonyl CoA 2 carbon units are added to the growing fatty acid with elongation terminated at a carbon chain length of 14 or less by the mammary gland-specific enzyme thioesterase II rather than at 16 carbons, as occurs in the liver and other tissues (7, 8). Synthesis and secretion of 10:0, 12:0, and 14:0 into milk is increased in lactating women consuming high-carbohydrate diets, whereas the secretion of the 18 carbon chain unsaturated fatty acids, which are derived by uptake from plasma, is decreased (4, 9, 10). Overall, reciprocal changes in mammary gland-derived medium-chain fatty acids (MCFA) and plasma-derived unsaturated fatty acids allows the milk fat content to be maintained under conditions of varying maternal dietary fat and carbohydrate intake. The levels of unsaturated fatty acids, including 18:1(n-9), 18:2(n-6), 18:3(n-3), 20:5(n-3), 22:6(n-3), and trans fatty acids in human milk, however, vary widely, with the maternal dietary fat composition being one of the most important factors contributing to the differences in the levels of unsaturated fatty acids in the milk of different women (4, 8, 11–14). In contrast, the levels of 16:0 in milk from women in different countries and with different diets is relatively constant at 20–25% of the milk fatty acids regardless of differences in the maternal diet fat content or composition (4). Possible exceptions include lower levels of 14–18% 16:0 described for milk from women in Gambia (15), some vegans and vegetarians (16, 17), and the Arctic Inuit (18).”
2. Evidence type: review of experiments in humans and rats
Lactate utilization by brain cells and its role in CNS development.
Medina JM, Tabernero A.
J Neurosci Res. 2005 Jan 1-15;79(1-2):2-10.
“Striking changes in the fuel supply to the tissues occur during the perinatal period because the transplacental supply of nutrients ends with a period of postnatal starvation (presuckling period) followed by adaptation to a fat-rich diet.”
[…]
“Ketone bodies are a major fuel for the brain during the suckling period and hence the stimulation of ketogenesis at birth is an important metabolic event in adaptation of the newborn to extrauterine life. Ketogenesis is active during late gestation in human fetal liver and the activity of ketogenic enzymes sharply increases immediately after birth in the rat (Hahn and Novak, 1985; Bougneres et al., 1986). In addition to modulation of enzyme activities, the control of ketogenesis also depends on the availability of fatty acids. The increase in fatty acid concentrations that occurs after delivery is due to breakdown of triacylglycerol in white adipose tissue present in human newborns at birth. In the rat, however, plasma fatty acids mostly come from hydrolysis of triacylglycerols from the mother’s milk because of the lack of white adipose tissue at birth. Nevertheless, in both species, once lactation is active fatty acids come from the intestinal hydrolysis of milk triacylglycerols, which may be absorbed directly without passage through the lymph (Aw and Grigor, 1980).”
[…]
“The increase in the activities of ketogenic enzymes together with the increase in the availability of fatty acids occurring immediately after delivery result in enhancement of ketogenic capacity of the liver (Girard,1990). This is responsible for the increase in ketone body concentrations observed postnatally. In fact, plasma ketone body concentrations are the main factor controlling the rate of ketone body utilization by neonatal tissues (Robinson and Williamson, 1980). In addition, activities of enzymes involved in ketone body utilization either increase during the first days of extrauterine life, as in the rat (Page et al., 1971), or are already induced during early gestation, as in the human brain (Patel et al., 1975). Moreover, newborn rat brain contains acetoacetyl-CoA synthetase, a unique enzyme that allows an important portion of carbon atoms from ketone bodies to be incorporated into lipid via a highly efficient cytosolic pathway (Williamson and Buckley, 1973). Indeed, there is a strong correlation between lipid synthesis and the activity of this enzyme during brain development (Yeh and Sheehan, 1985). Moreover, ketone body transport across the blood–brain barrier using the monocarboxylate carrier is maximal during the suckling period, in keeping with the idea that ketone bodies play an important role in brain development (Cremer, 1982; Conn et al., 1983).
“Ketone bodies are utilized by the newborn brain as a source of energy and carbon skeletons and are incorporated into fatty acids, sterols, acetylcholine, and amino acids (Robinson and Williamson, 1980; Bougneres et al., 1986). Ketone bodies, however, seem to be the major source of carbon skeletons for sterol synthesis during brain development and play a decisive role in the synthesis of brain structures during myelinogenesis (Robinson and Williamson, 1980; Miziorko et al., 1990). Ketone bodies are utilized evenly by neurons, astrocytes, and oligodendrocytes (Edmond et al., 1987; Lopes-Cardozo et al., 1989; Poduslo and Miller, 1991), indicating that they are ubiquitous substrates for brain cells. Acetoacetyl-CoA synthetase activity, however, is higher in oligodendrocytes than in neurons or astrocytes, confirming the special role of oligodendrocytes in myelinogenesis (Pleasure et al., 1979; Lopes-Cardozo et al., 1989; Poduslo and Miller, 1991).”
3. Evidence type: review of experiments
Metabolic adaptation at birth.
Ward Platt M, Deshpande S.
Semin Fetal Neonatal Med. 2005 Aug;10(4):341-50.
“During the first 8 h after birth, newborn infants have been shown to have rather low plasma ketone body concentrations despite adequate levels of precursor free fatty acids (FFAs), reflecting limited capacity for hepatic ketogenesis.30 Thereafter, from 12 h of age, healthy term infants show high ketone body turnover rates (12e 22 mmol kg/min) approaching those found in adults after several days of fasting,14 and during days 2 and 3 after birth they exhibit high ketone body concentrations quantitatively similar to those observed after an overnight fast in older children (Fig. 2).15 Such ketone body concentrations may account for as much as 25% of the neonate’s basal energy requirements during this time. Thus vigorous ketogenesis appears to be an integral part of extrauterine metabolic adaptation in the term human neonate.”
4. Evidence type: review of experiments in humans and rats
Lactate utilization by brain cells and its role in CNS development.
Medina JM, Tabernero A.
J Neurosci Res. 2005 Jan 1-15;79(1-2):2-10.
“Although the supply of metabolic substrates is maintained mostly during the perinatal period, there is an apparent lack of mobilization of energy reserves immediately after delivery; i.e., during the presuckling period. During this period, the maternal supply of glucose has ceased and alternative substrates have not yet been released. In the rat, fatty acids come exclusively from the mother’s milk because of the lack of white adipose tissue at birth. Consequently, free fatty acids are not available in the rat before the onset of suckling (Mayor and Cuezva, 1985; Girard, 1990). In the case of human newborns, however, fatty acid mobilization occurs immediately after birth, although the onset of ketogenesis is delayed, probably as a consequence of a limited supply of carnitine, which is provided mainly by the milk (Hahn and Novak, 1985; Schmidt-Sommerfeld and Penn, 1990). In addition, glycogenolysis and gluconeogenesis are not active immediately after birth, resulting in very low concentrations of plasma glucose (Mayor and Cuezva, 1985; Girard, 1990). In these circumstances, lactate may play an important role as an alternative substrate. In fact, lactate accumulates in fetal blood during the perinatal period and is removed rapidly immediately after delivery (Persson and Tunell, 1971; Juanes et al., 1986).”
5. Evidence type: presumably this is a review.
We could not get the full text, so for us this is evidence by authority
Ketone body metabolism in the mother and fetus.
Shambaugh GE 3rd.
Fed Proc. 1985 Apr;44(7):2347-51.
“Pregnancy is characterized by a rapid accumulation of lipid stores during the first half of gestation and a utilization of these stores during the latter half of gestation. Lipogenesis results from dietary intake, an exaggerated insulin response, and an intensified inhibition of glucagon release. Increasing levels of placental lactogen and a heightened response of adipose tissue to additional lipolytic hormones balance lipogenesis in the fed state. Maternal starvation in late gestation lowers insulin, and lipolysis supervenes. The continued glucose drain by the conceptus aids in converting the maternal liver to a ketogenic organ, and ketone bodies produced from incoming fatty acids are not only utilized by the mother but cross the placenta where they are utilized in several ways by the fetus: as a fuel in lieu of glucose; as an inhibitor of glucose and lactate oxidation with sparing of glucose for biosynthetic disposition; and for inhibition of branched-chain ketoacid oxidation, thereby maximizing formation of their parent amino acids. Ketone bodies are widely incorporated into several classes of lipids including structural lipids as well as lipids for energy stores in fetal tissues, and may inhibit protein catabolism. Finally, it has recently been shown that ketone bodies inhibit the de novo biosynthesis of pyrimidines in fetal rat brain slices. Thus during maternal starvation ketone bodies may maximize chances for survival both in utero and during neonatal life by restraining cell replication and sustaining protein and lipid stores in fetal tissues.”
Lipid Metabolism in Pregnancy and its Consequences in the Fetus and Newborn
Herrera, Emilio
Endocrine, Volume 19, Number 1, October 2002 , pp. 43-56(14)
“During early pregnancy there is an increase in body fat accumulation, associated with both hyperphagia and increased lipogenesis. During late pregnancy there is an accelerated breakdown of fat depots, which plays a key role in fetal development. Besides using placental transferred fatty acids, the fetus benefits from two other products: glycerol and ketone bodies. Although glycerol crosses the placenta in small proportions, it is a preferential substrate for maternal gluconeogenesis, and maternal glucose is quantitatively the main substrate crossing the placenta. Enhanced ketogenesis under fasting conditions and the easy transfer of ketones to the fetus allow maternal ketone bodies to reach the fetus, where they can be used as fuels for oxidative metabolism as well as lipogenic substrates.”
[…]
“Increased gluconeogenesis from glycerol and ketogenesis from NEFA may benefit the fetus, which at late gestation is at its maximum accretion rate and its requirements for substrates and metabolic fuels are greatly augmented. The preferential use of glycerol for gluconeogenesis and the efficient placental transfer of the newly formed glucose may be of major importance to the fetus under these fasting conditions (Fig. 2), in which the availability of other essential substrates such as amino acids is reduced (30,34). Placental transfer of ketone bodies is highly efficient (35), reaching fetal plasma at the same level as in maternal circulation (29). Ketone bodies may be used by the fetus as fuels (36) and as substrates for brain lipid synthesis (37).”
7. Evidence type: review
We could not get the full text, so for us this is evidence by authority
Gluconeogenesis in late fetal and early neonatal life.
Girard J.
Biol Neonate. 1986;50(5):237-58.
“Abstract
Birth in most mammalian species represents an abrupt change from a high-carbohydrate and low-fat diet to a high-fat and low-carbohydrate diet. Gluconeogenesis is absent from the liver of the fetus of well fed mothers, but can be induced prematurely by prolonged fasting of the mother. Gluconeogenesis increases rapidly in the liver of newborn mammals in parallel with the appearance of phosphoenolpyruvate carboxykinase (PEPCK), the rate-limiting enzyme of this pathway. The rise in plasma glucagon and the fall in plasma insulin which occur immediately after birth are the main determinants of liver PEPCK induction. When liver PEPCK has reached its adult value, i.e. 24 h after birth, other factors are involved in the regulation of hepatic gluconeogensis. In order to maintain a high gluconeogenic rate, the newborn liver must be supplied with sufficient amount of gluconeogenic substrates and free fatty acids. An active hepatic fatty acid oxidation is necessary to support hepatic gluconeogenesis by providing essential cofactors such as acetyl CoA and NADH. The relevance of animal studies for the understanding of neonatal glucose homeostasis in man is discussed.”
8. Evidence type: review of experiments:
Gluconeogenesis in the fetus and neonate.
Kalhan S, Parimi P.
Semin Perinatol. 2000 Apr;24(2):94-106.
(emphasis ours)
“Studies in human and animal models have consistently confirmed the dependence of the fetus on the mother for supply of glucose so that the fetus in utero under normal physiological circumstances does not produce glucose. However, most gluconeogenic and glycogenolytic enzymes have been shown to be present early in fetal development. The exception is the cytosolic phosphoenol pyruvate carboxykinase, which is expressed (at least in the rat) immediately after birth. 12-14 The appearance of gluconeogenic enzyme activity in the liver in relation to birth in the rat fetus and newborn is displayed in Figure 2. As shown, PC and glucose-6-phosphatase activity are expressed in the fetus, are relatively low at birth, and increase rapidly thereafter. Fructose 1,6-diphosphatase activity increases before birth. In contrast, phosphoenol pyruvate carboxykinase activity is absent in the fetus and rapidly increases immediately after birth, so that hepatic gluconeogenesis is completely absent in utero and appears in the immediate newborn period 2,14,5 GNG, however, can,be induced in utero by prolonged maternal starvation, prolonged hypoglycemia in the mother, or by direct injection of cyclic adenosine monophosphate (cAMP) into the fetus. 16-18 In addition, some studies have showed incorporation of tracer carbon from lactate into glucose in rat fetus and glutamine carbon into hepatic glycogen in sheep fetus. 4,5,19 The significance of these latter observations remains unclear.”
9. Evidence type: meta-analysis
Breast milk and cognitive development–the role of confounders: a systematic review.
Walfisch A, Sermer C, Cressman A, Koren G.
BMJ Open. 2013 Aug 23;3(8):e003259. doi: 10.1136/bmjopen-2013-003259.
“The association between breastfeeding and child cognitive development is conflicted by studies reporting positive and null effects. This relationship may be confounded by factors associated with breastfeeding, specifically maternal socioeconomic class and IQ.
Design
Systematic review of the literature.
Setting and participants
Any prospective or retrospective study, in any language, evaluating the association between breastfeeding and cognitive development using a validated method in healthy term infants, children or adults, was included.
Primary and secondary outcome measures
Extracted data included the study design, target population and sample size, breastfeeding exposure, cognitive development assessment tool used and participants’ age, summary of the results prior to, and following, adjustment for confounders, and all confounders adjusted for. Study quality was assessed as well.
Results
84 studies met our inclusion criteria (34 rated as high quality, 26 moderate and 24 low quality). Critical assessment of accepted studies revealed the following associations: 21 null, 28 positive, 18 null after adjusting for confounders and 17 positive—diminished after adjusting for confounders. Directionality of effect did not correlate with study quality; however, studies showing a decreased effect after multivariate analysis were of superior quality compared with other study groupings (14/17 high quality, 82%). Further, studies that showed null or diminished effect after multivariate analysis corrected for significantly more confounders (7.7±3.4) as compared with those that found no change following adjustment (5.6±4.5, p=0.04). The majority of included studies were carried out during childhood (75%) and set in high-income countries (85.5%).
Conclusions
Much of the reported effect of breastfeeding on child neurodevelopment is due to confounding. It is unlikely that additional work will change the current synthesis. Future studies should attempt to rigorously control for all important confounders. Alternatively, study designs using sibling cohorts discordant for breastfeeding may yield more robust conclusions.”
10. Evidence type: observational
Infant feeding and mental and motor development at 18 months of age in first born singletons.
Florey CD, Leech AM, Blackhall A.
Int J Epidemiol. 1995;24 Suppl 1:S21-6.
“OBJECTIVE:
To determine the relationship between type of infant feeding and mental and psychomotor development at age 18 months.
METHOD:
A follow-up study of children born to primigravidae living in Dundee and booked into antenatal clinics in the City of Dundee (Local Authority District) from 1 May 1985 to 30 April 1986. The study population was 846 first born singletons, of whom 592 attended for developmental assessment at age 18 months. The main outcome measures were the Bayley Scales of Infant Mental and Motor Development.
RESULTS:
Higher mental development was significantly related to breast feeding on discharge from hospital and according to the health visitors’ notes at about 2 weeks after discharge after allowing for partner’s social class, mother’s education, height, alcohol and cigarette consumption; placental weight and the child’s sex, birth weight and gestational age at birth. After adjustment for statistically significant variables, the difference in Bayley mental development index between breast and bottle fed infants was between 3.7 and 5.7 units depending on the source of feeding data. No differences were found for psychomotor development or behaviour.
CONCLUSION:
The study provides further evidence of a robust statistical association between type of feeding and child intelligence. However, the literature is replete with suggestions for potential confounding variables which offer alternative causal explanations. To unravel what is an important clinical and public health question, further research should concentrate on randomized trials of supplemented formula feeds for children of mothers opting for bottle feeding and on epidemiological studies designed to disentangle the relation between method of feeding, parental intelligence and social environment.”
11. Evidence type: observational
Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity.
Belfort MB, Rifas-Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, Gillman MW, Oken E.
JAMA Pediatr. 2013 Sep;167(9):836-44.
“Breastfeeding may benefit child cognitive development, but few studies have quantified breastfeeding duration or exclusivity, nor has any study to date examined the role of maternal diet during lactation on child cognition.
OBJECTIVES:
To examine relationships of breastfeeding duration and exclusivity with child cognition at ages 3 and 7 years and to evaluate the extent to which maternal fish intake during lactation modifies associations of infant feeding with later cognition.
DESIGN, SETTING, AND PARTICIPANTS:
Prospective cohort study (Project Viva), a US prebirth cohort that enrolled mothers from April 22, 1999, to July 31, 2002, and followed up children to age 7 years, including 1312 Project Viva mothers and children.
MAIN EXPOSURE:
Duration of any breastfeeding to age 12 months.
MAIN OUTCOMES AND MEASURES:
Child receptive language assessed with the Peabody Picture Vocabulary Test at age 3 years, Wide Range Assessment of Visual Motor Abilities at ages 3 and 7 years, and Kaufman Brief Intelligence Test and Wide Range Assessment of Memory and Learning at age 7 years.
RESULTS:
Adjusting for sociodemographics, maternal intelligence, and home environment in linear regression, longer breastfeeding duration was associated with higher Peabody Picture Vocabulary Test score at age 3 years (0.21; 95% CI, 0.03-0.38 points per month breastfed) and with higher intelligence on the Kaufman Brief Intelligence Test at age 7 years (0.35; 0.16-0.53 verbal points per month breastfed; and 0.29; 0.05-0.54 nonverbal points per month breastfed). Breastfeeding duration was not associated with Wide Range Assessment of Memory and Learning scores. Beneficial effects of breastfeeding on the Wide Range Assessment of Visual Motor Abilities at age 3 years seemed greater for women who consumed 2 or more servings of fish per week (0.24; 0.00-0.47 points per month breastfed) compared with less than 2 servings of fish per week (−0.01; −0.22 to 0.20 points per month breastfed) (P = .16 for interaction).
CONCLUSIONS AND RELEVANCE:
Our results support a causal relationship of breastfeeding duration with receptive language and verbal and nonverbal intelligence later in life.”
12. Evidence type: observational
A cohort study on full breastfeeding and child neuropsychological development: the role of maternal social, psychological, and nutritional factors.
Julvez J, Guxens M, Carsin AE, Forns J, Mendez M, Turner MC, Sunyer J.
Dev Med Child Neurol. 2014 Feb;56(2):148-56. doi: 10.1111/dmcn.12282. Epub 2013 Oct 1.
“AIM:
This study investigated whether duration of full breastfeeding is associated with child neuropsychological development and whether this association is explained by social, psychological, and nutritional factors within families.
METHOD:
Participants in this study were a population-based birth cohort in the city of Sabadell (Catalonia, Spain). Females were recruited during the first trimester of pregnancy between July 2004 and July 2006. Information about parental characteristics and breastfeeding was obtained through questionnaires. Full breastfeeding was categorized as never, short term (≤4mo), long term (4-6mo), or very long term (>6mo). A trained psychologist assessed the neuropsychological development of children at 4 years of age (n=434) using the McCarthy Scales of Children’s Abilities (MSCA).
RESULTS:
Full breastfeeding showed an independent association with child general MSCA scores after adjusting for a range of social, psychological, and nutritional factors (>6mo, coefficient=7.4 [95% confidence interval=2.8-12.0], p=0.011). Maternal social class, education level, and IQ were also associated with child neuropsychological scores, but did not explain breastfeeding associations. Omega-3 (n3) fatty acid levels were not associated with child neuropsychological scores.
INTERPRETATION:
Very long-term full breastfeeding was independently associated with neuropsychological functions of children at 4 years of age. Maternal indicators of intelligence, psychopathology, and colostrum n3 fatty acids did not explain this association.”
13. Evidence type: observational
Breast feeding and allergic diseases in infants—a prospective birth cohort study
I Kull, M Wickman, G Lilja, S Nordvall, and G Pershagen
Arch Dis Child. 2002 December; 87(6): 478–481. doi: 10.1136/adc.87.6.478 PMCID: PMC1755833
“Aims: To investigate the effect of breast feeding on allergic disease in infants up to 2 years of age.
Methods: A birth cohort of 4089 infants was followed prospectively in Stockholm, Sweden. Information about various exposures was obtained by parental questionnaires when the infants were 2 months old, and about allergic symptoms and feeding at 1 and 2 years of age. Duration of exclusive and partial breast feeding was assessed separately. Symptom related definitions of various allergic diseases were used. Odds ratios (OR) and 95% confidence intervals (CI) were estimated in a multiple logistic regression model. Adjustments were made for potential confounders.
Results: Children exclusively breast fed during four months or more exhibited less asthma (7.7% v 12%, ORadj = 0.7, 95% CI 0.5 to 0.8), less atopic dermatitis (24% v 27%, ORadj = 0.8, 95% CI 0.7 to 1.0), and less suspected allergic rhinitis (6.5% v 9%, ORadj = 0.7, 95% CI 0.5 to 1.0) by 2 years of age. There was a significant risk reduction for asthma related to partial breast feeding during six months or more (ORadj = 0.7, 95% CI 0.5 to 0.9). Three or more of five possible allergic disorders—asthma, suspected allergic rhinitis, atopic dermatitis, food allergy related symptoms, and suspected allergic respiratory symptoms after exposure to pets or pollen—were found in 6.5% of the children. Exclusive breast feeding prevented children from having multiple allergic disease (ORadj = 0.7, 95% CI 0.5 to 0.9) during the first two years of life.
Conclusion: Exclusive breast feeding seems to have a preventive effect on the early development of allergic disease—that is, asthma, atopic dermatitis, and suspected allergic rhinitis, up to 2 years of age. This protective effect was also evident for multiple allergic disease.”
14. Evidence type: review of observational studies
Does breastfeeding influence the risk of developing diabetes mellitus in children? A review of current evidence.
Pereira PF, Alfenas Rde C, Araújo RM.
J Pediatr (Rio J). 2014 Jan-Feb;90(1):7-15. doi: 10.1016/j.jped.2013.02.024. Epub 2013 Oct 16.
“Objective
the aim of this study was to perform a review to investigate the influence of breastfeeding as a protective agent against the onset of diabetes in children.
Sources
non-systematic review of SciELO, LILACS, MEDLINE, Scopus, and VHL databases, and selection of the 52 most relevant studies. A total of 21 articles, specifically on the topic, were analyzed (nine related to type 1 diabetes and 12 to type 2 diabetes).
Data synthesis
the duration and exclusivity of breastfeeding, as well as the early use of cow’s milk, have been shown to be important risk factors for developing diabetes. It is believed that human milk contains substances that promote the maturation of the immune system, which protect against the onset of type 1 diabetes. Moreover, human milk has bioactive substances that promote satiety and energy balance, preventing excess weight gain during childhood, thus protecting against the development of type 2 diabetes. Although the above mentioned benefits have not been observed by some researchers, inaccuracies on dietary habit reports during childhood and the presence of interfering factors have been considered responsible for the lack of identification of beneficial effects.
Conclusion
given the scientific evidence indicated in most published studies, it is believed that the lack of breastfeeding can be a modifiable risk factor for both type 1 and type 2 diabetes. Strategies aiming at the promotion and support of breastfeeding should be used by trained healthcare professionals in order to prevent the onset of diabetes.”
15. Evidence type: observational
Breastfeeding and risk of epilepsy in childhood: a birth cohort study.
Sun Y, Vestergaard M, Christensen J, Olsen J.
J Pediatr. 2011 Jun;158(6):924-9. doi: 10.1016/j.jpeds.2010.11.035. Epub 2011 Jan 13.
“OBJECTIVE:
We asked whether breastfeeding reduces the risk of epilepsy in childhood.
STUDY DESIGN:
We included 69 750 singletons born between September 1997 and June 2003 in the Danish National Birth Cohort and observed them to August 2008. Information on breastfeeding was reported by mothers in two computer-assisted telephone interviews at 6 and 18 months after birth. Information on epilepsy (inpatients and outpatients) was retrieved from the Danish National Hospital Register. Cox proportional hazards regression models were used to estimate incidence rate ratios and 95% CIs.
RESULTS:
Breastfeeding was associated with a decreased risk of epilepsy, with a dose-response like pattern. For example, children breastfed for 3 to 5, 6 to 8, 9 to 12, and ≥ 13 months had a 26%, 39%, 50%, and 59% lower risk of epilepsy after the first year of life, respectively, compared with children who were breastfed for <1 month. The association remained when we excluded children who had adverse neonatal conditions or children who were exposed to adverse maternal conditions during pregnancy.
CONCLUSIONS:
The observed protective effect of breastfeeding may be causal. Breastfeeding may decrease epilepsy in childhood, thereby adding another reason for breastfeeding.”
16. Evidence type: controlled human experiments
Nutritional factors that affect the postnatal metabolic adaptation of full-term small- and large-for-gestational-age infants.
de Rooy L, Hawdon J.
Pediatrics. 2002 Mar;109(3):E42.
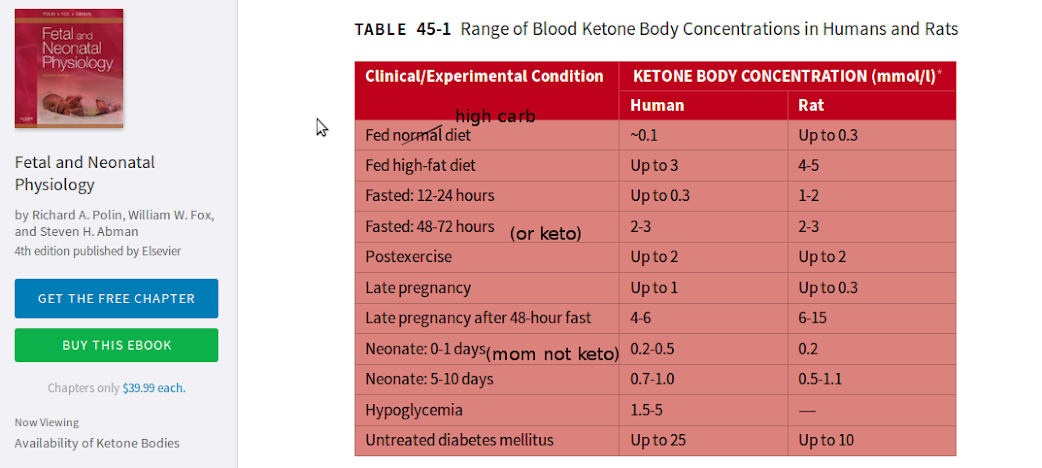
“Our summary statistic, median peak kb [(ketone body)] concentration (Table 6), is significantly higher in the BF [(breastfed)] group compared with other feed groups for the SGA [(small for gestational age)] infants analyzed separately. We further explored the relationship between the blood glucose concentration and kb response by finding the kb concentration at the lowest blood glucose level for each infant at >24 hours of age (Fig 3, Table 6). Especially at low blood glucose values, infants who receive breast milk show some of the highest values for blood kb concentration. Our data show that exclusive formula feeding does not necessarily protect against low blood glucose values. Hence, the SGA FF [(formula fed)] infant could be doubly at risk of both low blood glucose values with a reduced kb response. No BF infant had both low blood glucose and low kb levels. For LGA [(large for gestational age)] infants, low blood glucose values were offset by kb concentrations of the same order of magnitude previously demonstrated for AGA [(appropriate for getstaional age)] infants6 (Fig 3).”
[…]
“Mammalian animal studies have shown that the postnatal induction of the enzymes involved in β-oxidation within the mitochondria requires the presence of long-chain fatty acids.15 The carnitine palmitoyltransferase system, which controls movement of long-chain fatty acids into the mitochondria, represents a major rate-limiting step in ketogenesis in the suckling rat. Long-chain fatty acids play a pivotal role in the posttranscriptional regulation of carnitine palmitoyltransferase 1 during the immediate postnatal period. We speculate that a factor present in breast milk but absent in formula milk augments ketogenesis in human neonates in the same way. Carnitine is known to have a central role in β-oxidation of fats: it is responsible for the transport of fatty acyl-coenzyme A across the inner mitochondrial membrane.16 During the suckling period, the demand for carnitine exceeds the rate of endogenous synthesis by up to 50%.17 Indeed, healthy, full-term infants fed formulas devoid of carnitine showed reduction in ketogenesis and an accumulation of fatty acid precursors in the plasma. Although breast milk– and cow’s milk– derived formulas contain equivalent amounts of carnitine,18 it may well be that there are significant differences in bioavailability. When compared with breastfed control subjects, infants who were fed a standard formula that was not supplemented with carnitine demonstrated markers of carnitine deficiency.19 Furthermore, we hypothesized that high intakes of energy and protein associated with early formula feeding may “switch off” or dampen the crucial glucagon surge, central to regulation of fuel availability in the immediate postnatal period.”