Protein, Gluconeogenesis, and Blood Sugar
Recently (for some conception of recent) we asked the question: If You Eat Excess Protein, Does It Turn Into Excess Glucose?
One of the potentially confusing aspects of this question, is the difference between gluconeogenesis (GNG) — the creation of new glucose that didn't exist before, and increases in blood sugar. In response to our post, several people made comments that indicated an implicit assumption that changes in blood sugar can be used as a measurement of GNG, but as we will explain below, this is not the case. However, it brought to our attention an important distinction.
There are several reasons people might care about excess GNG. One we have already addressed: It is not the case that GNG requires excess cortisol.
In terms of the effect of the glucose itself that results from GNG, there are two distinct concerns:
- How does excess GNG affect blood sugar levels? Blood sugar levels are important because too much sugar in the blood at a given time can cause damage to cells ⁰.
- Does producing more glucose via GNG ultimately lead to either using more glucose for fuel, or storing it as fat?
So when people worry about protein causing excess GNG, what they are really worrying about is that protein will adversely affect their blood sugar levels, or that they are going to use more glucose for fuel than they intended, or that they will store it as unwanted fat.
While it would be interesting to understand the effect of eating excess protein on GNG, it doesn't directly address those underlying questions, because there are many other mechanisms in play. We want to know whether for ketogenic dieters eating excess protein adversely affects blood sugar levels, whether it leads to higher consumption of glucose for fuel, and whether it increases the tendency to store fat.
In this article, we will directly approach the first of these questions.
In Brief:
- Because the level of sugar in your blood depends on how much is coming in (and there is more than one source), and how much is going out, changes in blood sugar cannot by themselves tell us about the rate of GNG.
- Nonetheless, when we wrote the original article suggesting that eating excess protein was unlikely to result in an increased rate of GNG, one of the assumptions we made was that eating protein does not raise blood sugar. We made this assumption because it is true in non-diabetic, non-ketogenic dieters. However, we have recently learned that this is not true in ketogenic dieters.
- In response to protein, blood sugar rises on a keto diet, even though I/G — the ratio of insulin to glucagon, stays constant, whereas on a glycolytic diet, I/G rises, but blood sugar stays constant. There is evidence that I/G is tightly correlated with glucose production, so this is suggestive that in keto dieters glucose production is not affected by eating protein. However, we lack direct experimental evidence.
- Although blood sugar rises in keto dieters eating protein, it stays within safe bounds, at least in our experience, and in the experiment we looked at.
What affects blood sugar?
Fundamentally, the amount of sugar in the blood at any given time depends on two things: how much is coming in, and how much is going out. On the input side, blood sugar can come from three sources:
- We can eat carbohydrates, and have sugar enter the blood through digestion.
- We can make glucose out of glycogen (the limited amount of glucose stored in persistent form in the liver). This process is called glycogenolysis.
- Thirdly, we can produce new glucose by GNG.
Note that because of glycogen storage, it is possible for sugar to enter the blood that has not come directly from GNG. Even on a keto diet, there is still a substantial proportion of glucose production from glycogenolysis. Ultimately, of course, the glycogen in keto dieters also comes from GNG that happened previously.
On the outgoing side, the rate of sugar leaving the blood can also change depending on the uptake by cells. It can be used for fuel, stored as fat, or turned into glycogen for storage (glycogenesis).
This means that changes in blood sugar can happen in either direction, without us being able to conclude anything about the rate of GNG. Therefore, observations about increases in blood sugar in response to protein are not conclusive evidence about GNG.
The effect of protein on blood sugar
It is well established that under typical test conditions protein ingestion does not significantly effect blood sugar levels, either alone ¹ or in combination with other foods ². This graph shows the typical response to glucose (solid line) and protein (dashed line) ingestion in two non-diabetics after an overnight fast ³:
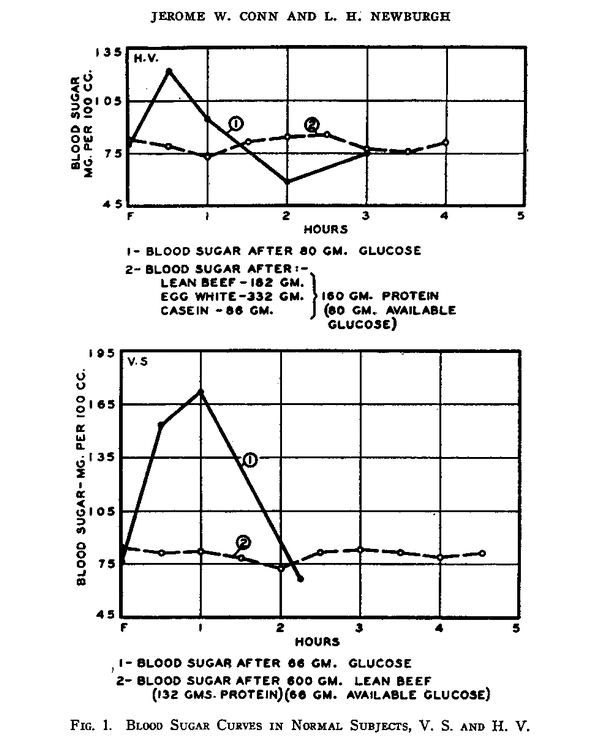
Of course, the “typical conditions” in those experiments did not include keto dieters.
However, there was an experiment in 1971 that did test the blood glucose response to protein in people who had restricted carbohydrates to ketogenic levels for one week, and compared it to the values after reintroducing carbohydrates for a week ⁴. The group size was small, and the keto-adaptation time was short, but nonetheless, the results showed that blood glucose did in fact rise in the keto dieters ingesting protein. The experimenters also measured the blood levels of the blood sugar regulating hormones insulin and glucagon and their ratio, I/G.
There is evidence that it is I/G, and not the absolute levels of either hormone, that ultimately regulates glucose production ⁵,⁶,⁷,⁸.
Interestingly, in the keto dieters, although the levels of these hormones significantly changed, their ratio did not.
Here is a summary of the results:
| Diet type | After |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| insulin | I/G |
I/G |
||||||||||||||
| 78 +/- 3 | 8 +/- 2 | 128 +/- 17 | 1.7 +/- 0.3 | 90 +/- 3 | 15 +/- 4 | 218 +/- 10 | 1.83 +/- 0.4 | |||||||||
| Not ketogenic | 94 +/- 5 | 17 +/- 4 | 87 +/- 15 | 4.35 +/- 1.38 | 28 +/- 14 | 167 +/- 13 | 8.2 +/- 4.66 | |||||||||
In response to protein, blood sugar rises on a keto diet, even though I/G stays constant, whereas on a glycolytic diet, I/G rises, but blood sugar stays constant.
Because the number of subjects was small, the authors emphasize that this study cannot give us precise estimates of the numerical values of blood sugar (or hormones) in response to protein. It can only tell us that this rise in blood sugar happens qualitatively. In other words, they've shown that a rise in blood sugar happens, but we can't be sure that the average rise is going to be 10 mg/dL if we tested a lot more people.
It is important to understand that this is not a very complete picture. For example, it doesn't show whether there is a relationship between how much protein is eaten and blood sugar. It could be, for example, that any amount of protein results in the same increase in blood sugar. It could be that protein ingestion results in a small rise in blood sugar with a duration that depends on the amount of protein. It could be that eating protein on a keto diet causes your blood sugar to rise steadily according to the amount of protein eaten until it reaches some maximum value, such as a 20 point rise, after which no further amount of protein has an effect. What is very unlikely is that the amount of protein has an unlimited effect: we would not expect, for example that if eating 50g of protein caused a 10 point rise, then eating 300g of protein would cause a 60 point rise. There is still a lot to learn here.
| Note: Incidentally, these experiments would be easy for any of us to do at home. For example, you could get up after an overnight fast and measure your blood sugar. Then ingest 25g of protein, and measure your blood sugar every half hour until it returns to baseline to see how your blood sugar changes and for how long. Do that for three days, and then try it with 50g for three days. Then try 100g and then 200g. Use the same kind of protein in each case, like lean beef or egg whites (save the yolks for later!). Tell us what you find out. |
One important conclusion from all of this, though, is that, at least at these protein levels, blood sugar stays in a safe range ⁹. There is good reason to be concerned about the level of sugar in your blood over the long term. We will write more about that in subsequent posts. For now we can simply note that the fasting level of blood sugar in this experiment was at the low end of the range considered optimal, and the rise in response to protein was well below the amount considered dangerous.
Acknowledgements:
This article was most spurred by a comment on the original article by Anna K. Thank you, Anna.
Once again, we are indebted to friendly correspondents for access to relevant scientific papers. This article was supported by friends in Windy City, Orlando, and Edinburgh. We couldn't have written it without you!
References:
0. Evidence type: summary of experiments
(Emphasis ours)
Type 2 diabetes mellitus (DM) is a progressive disease characterized by elevated plasma glucose levels. Type 2 DM results from a combination of factors affecting both peripheral tissue insulin sensitivity and β-cell function. A survey of the scientific literature on DM, glucose toxicity, hyperglycemia, nephropathy, neuropathy, reactive oxygen species, and retinopathy cited on PubMed/Medline from January 1975 to May 2011 was conducted. The relevant publications, chosen at the author's discretion, were used to synthesize this narrative review article. Chronic hyperglycemia imposes damage (glucose toxicity) on a number of cell types and is strongly correlated with the myriad of DM-related complications. Tissues most vulnerable to the effects of prolonged elevated plasma glucose levels include pancreatic β cells and vascular endothelial cells. The ensuing β-cell dysfunction promotes decreased insulin synthesis and secretion, further perpetuating the associated hyperglycemia. As for the vascular endothelium, chronic hyperglycemia is strongly correlated with many DM-related microvascular complications, including retinopathy, nephropathy, and neuropathy. The role of hyperglycemia in macrovascular complications is not well defined. Pathophysiologic modifications that arise in response to chronic hyperglycemia persist and may promote DM-related complications that manifest years later, even if plasma glucose levels have been brought under control. Increasing awareness of the mechanisms by which even modest hyperglycemia promotes long-lasting tissue damage highlights the need to achieve early tight glycemic control in patients with DM before substantial disease progression.
1. Evidence type: review of experiments
"Protein has a minimal effect on blood glucose levels with adequate insulin. However, with insulin deficiency, gluconeogenesis proceeds rapidly and contributes to an elevated blood glucose level. With adequate insulin, the blood glucose response in persons with diabetes would be expected to be similar to the blood glucose response in persons without diabetes. The reason why protein does not increase blood glucose levels is unclear. Several possibilities might explain the response: a slow conversion of protein to glucose, less protein being converted to glucose and released than previously thought, glucose from protein being incorporated into hepatic glycogen stores but not increasing the rate of hepatic glucose release, or because the process of gluconeogenesis from protein occurs over a period of hours and glucose can be disposed of if presented for utilization slowly and evenly over a long time period."
2. Evidence type: experiment
"When mixed meals are consumed, other food and macronutrients will be present. In this study, the results were similar to those observed in studies using isolated carbohydrates [6] and imply that other macronutrients had a negligible effect on the differential serum glucose and insulin responses. It has, in fact, been reported elsewhere that the amount and type of carbohydrate account for about 90% of the total variability in blood glucose response, whereas protein and fat in mixed meals scarcely contribute to the variance in blood glucose and insulin responses [1,2]."
3. Evidence type: experiment
4. Evidence type: experiment
5. Evidence type: experiment (non-human animal)
(Emphasis ours)
The levels of serum insulin, glucagon, and free fatty acids (FFA) and the tissue concentrations of hepatic cyclic AMP, long-chain acyl-CoA (LCA), adenine nucleotides, inorganic phosphate, the intermediates of the Embden-Meyerhof pathway, the citric acid cycle (including acetyl-CoA and free CoA), and the cytoplasmic and mitochondrial redox couples were determined in the rat 12, 24, and 48 h after food withdrawal and 5, 10, 20, 40, 60, and 120 min after the refeeding of glucose. Using the measured metabolite contents in the liver, the alterations in the concentration of malate, oxaloacetate, citrate, and α-ketoglutarate and the changes in the energy state of the adenine nucleotide system and the redox state of the NAD system were attributed to the cytoplasmic and mitochondrial compartments by applying established calculation methods. Glucose refeeding provoked significant alterations in all parameters investigated. These changes occurred within minutes, reversing the hormone and metabolite pattern which had developed within 24 h in response to food withdrawal. Particularly, glucose refeeding resulted in a drastic increase in the insulin/glucagon ratio. Simultaneously, the level of serum FFA and the concentration of LCA in the liver declined. The latter alteration was accompanied by an increase in the cytoplasmic and a decrease in the mitochondrial ATP/ADP x P ratios. Moreover, the redox state of the cytoplasmic NAD system was shifted toward the oxidized state. When the appropriate data were plotted against each other, highly significant correlations were obtained (i) between the insulin/glucagon ratio and the serum FFA concentration, (ii) between the serum FFA concentration and the concentration of hepatic LCA, (iii) between the hepatic LCA concentration and the cytoplasmic energy state, and (iv) between the cytoplasmic energy state and the redox state of the cytoplasmic NAD system. These findings are interpreted to support the hypothesis derived from experiments carried out in vitro that the insulin/glucagon ratio via the FFA-dependent concentration of hepatic LCA might affect the translocation of adenine nucleotides between the cytoplasmic and the mitochondrial compartment, thereby regulating the cytoplasmic energy state and the redox state of the cytoplasmic NAD system, consequently. Glucose refeeding provoked rapid coordinate changes in the concentration of the intermediates of both the citric acid cycle and the Embden-Meyerhof chain, indicating the altered substrate flow through these pathways. Those metabolites, known to modulate the activity of key regulatory enzymes in vitro, were analyzed with respect to their suggested regulatory function. As to the established shift from pyruvate carboxylation to pyruvate decarboxylation after glucose refeeding, the data revealed that the decrease in pyruvate carboxylase activity can be attributed to the decrease in the intramitochondrial ATP/ADP ratio and the simultaneous fall in acetyl-CoA concentration, while the coordinate increase in pyruvate dehydrogenase activity can be ascribed to the decline in the concentration of LCA and, consequently, in the ratios of ATP/ADP , NADH/NAD, and acetyl-CoA/CoA within the mitochondria. As for the citric acid cycle, increased citrate synthesis from acetyl-CoA and oxaloacetate was supported by the rapid drop in the concentration of the established inhibitor of citrate synthesis, LCA. In contrast, the concentration of succinyl-CoA, an inhibitor of the enzyme in vitro, remained practically constant, questioning its regulatory function under the present experimental conditions. In addition to the activation of citrate synthase, the coordinate activation of isocitrate dehydrogenase was indicated by the LCA-mediated decline in both the mitochondrial ATP/ADP and the NADH/NAD ratios. Glucose refeeding immediately reduced urea excretion to basal values. This alteration was preceded by a drastic fall in the tissue concentration of cyclic AMP, supporting the physiological role of the nucleotide in the control of hepatic gluconeogenesis. In contrast, the observed changes in the concentration of the effectory acting metabolites (ATP, AMP, fructose 1,6-diphosphate, citrate, and alanine) were incompatible with the suggested function of these intermediates in switching over the substrate flow through the Embden-Meyerhof pathway from gluconeogenesis to glycolysis. The results are discussed in reference to the known rapid stimulation of fatty acid biosynthesis in the liver and to the transfer of reducing equivalents by the different shuttles of the inner mitochondrial membrane. In summary, it can be concluded that the insulin/glucagon ratio in a moment-to-moment fashion controls the glucose balance across the liver by regulating hepatic intermediary metabolism via the concentration of both LCA and cyclic AMP.
6. Evidence type: experiment (non-human animal)
(Emphasis ours)
Plasma hormones, glucose and free fatty acids, liver glycogen and two key enzymes of glycolysis and gluconeogenesis were examined in adult rats during a 40-day period of high protein feeding. Plasma insulin fell within 1 day but returned to normal after 4 days. Glucagon changed more slowly, reaching a maximum on day 4 and declined to near the control value within 24 days. Consequently, the insulin to glucagon ratio was lower on days 1, 4 and 8 and was nearly normal on day 24. With respect to hepatic enzymes, phosphoenolpyruvate carboxykinase activity rose sharply on the 1st day and remained elevated for 40-day period; the L-isozyme of pyruvate kinase, although unchanged on the 1st day, decreased thereafter and from day 8 on represented 15--20% of control. Circadian variations in these parameters were also measured in rats adapted to the high protein diet. In such animals, the diurnal change in plasma hormones was less marked but tended to be inverted with respect to controls; the insulin/glucagon ratio was highest during daylight on high protein and in late night on the control diet. Over 24 hours, pyruvate kinase activity was related directly and phosphoenolpyruvate carboxykinase inversely to the hormone ratio. We concluded that in rats adapted to high protein, as in controls, the diurnal balance between glycolysis and gluconeogenesis is probably regulated by the same factor, namely the insulin/glucagon ratio.
7. Evidence type: authority
The insulin/glucagon (I/G) ratio is a key determinant of lipolysis, glycogenolysis, and gluconeogenesis [14,15].
(We followed the references given for this assertion, and did not understand how they supported it, so we consider this evidence as authority-based, at least for us.)
8. Evidence type: in vitro experiment
The effect of physiological concentrations of glucagon and insulin on glycogenolysis was studied in the presence and absence of substrates in isolated hepatocytes containing high glycogen. In the absence of substrates glucagon stimulated glycogenolysis at 10−14M concentration, and addition of 100 μunits of insulin partially inhibited glucagon stimulated glycogenolysis (10−14M to 10−11M). However, in the presence of substrates, insulin completely inhibited glucagon stimulated glycogenolysis (10−14M to 10−11M), indicating that molar glucagon and insulin ratios control carbohydrate metabolism in liver. Additional studies showed incorporation of amino acid into protein was linear for only 3 to 4 hr in cells containing low glycogen, whereas in cells containing high glycogen, incorporation was linear for 8 to 10 hr.
9. Evidence type: authority
Dissatisfying as it is, for now we will talk about safe as meaning not identified as correlating with current or subsequent development of diabetes.We will talk more about blood sugar in a subsequent post.
(Emphasis ours)
In nondiabetic individuals, fasting plasma glucose concentrations (i.e., following an overnight 8- to 10-h fast) generally range from 70 to 110 mg/dl.
The magnitude and time of the peak plasma glucose concentration depend on a variety of factors, including the timing, quantity, and composition of the meal. In nondiabetic individuals, plasma glucose concentrations peak ∼60 min after the start of a meal, rarely exceed 140 mg/dl, and return to preprandial levels within 2–3 h. Even though glucose concentrations have returned to preprandial levels by 3 h, absorption of the ingested carbohydrate continues for at least 5–6 h after a meal.
